
Founded in 1976, SOPHYSA was the pioneer in the development of adjustable neurosurgical valves to treat hydrocephalus. Introduced in 1984, the Sophy® valve was the first adjustable valve in the World allowing non-invasive adjustment of operating pressures, to match an individual patient's changing clinical needs.
Acquired in 1989 by the Japanese tkb Group, SOPHYSA expanded quickly in France, and abroad, gaining global recognition in the field of Neurosurgery.
In 2004, SOPHYSA launched the Polaris® valve, the first adjustable valve with a lock, specifically designed to resist unintentional changes in operating pressure – a milestone in adjustable valve safety. Polaris® offers the patient an unequalled security against the clinical risks associated with those dysadjustments.
In 2005, SOPHYSA introduced Pressio®,a user-friendly Intra-Cranial Pressure monitoring system for neurosurgery and neurotrauma, bringing the continuous intracranial pressure monitoring to a new level of performance and ease of use.
As a neurosurgery specialist, SOPHYSA manufactures and markets a wide range of neurosurgical implants for the treatment of hydrocephalus, including various types of valves, catheters, reservoirs and accessories, a range of ICP sensors and external drainage systems, as well as subcutaneous spinal access ports.
SOPHYSA markets its products worldwide through an international network of exclusive distributors. In France and Benelux, SOPHYSA products are marketed by direct sales representatives.

ICP Monitoring is a key element for patient management with acute brain damages. Over the last decades, the ICP monitoring has evolved from the simple average value calculated every hour to the analysis of the signal shape, its evolution in the time and the calculation of new indicators, all integrated in a multimodal monitoring.
Face to these new expectations, and in accordance with our commitment to develop ground-breaking solutions to improve user experience, our new ICP monitoring system Pressio®2 has been designed to bring:
USER FRIENDLINESS
ADVANCED FUNCTIONS
IMPROVED COMPATIBILITY
PRESSIO® 2 SYSTEM CONFIGURATION
The Pressio® 2 system offers the possibility to transfer data of intracranial pressure and temperature monitoring either to a patient monitor or to a computer for research purposes, depending on clinicians’ needs.
For each type of transfer, different accessories are needed

ICP Monitoring is a key element for patient management with acute brain damages. Over the last decades, the ICP monitoring has evolved from the simple average value calculated every hour to the analysis of the signal shape, its evolution in the time and the calculation of new indicators, all integrated in a multimodal monitoring.
Face to these new expectations, and in accordance with our commitment to develop ground-breaking solutions to improve user experience, our new ICP monitoring system Pressio®2 has been designed to bring:
USER FRIENDLINESS
ADVANCED FUNCTIONS
IMPROVED COMPATIBILITY
PRESSIO® 2 SYSTEM CONFIGURATION
The Pressio® 2 system offers the possibility to transfer data of intracranial pressure and temperature monitoring either to a patient monitor or to a computer for research purposes, depending on clinicians’ needs.
For each type of transfer, different accessories are needed

Sophysa is a French industry pioneer specialized in CSF Management, and Neuro Monitoring for Neuro ICU.
Over the last 40 years, in accordance to our commitment, we never stopped challenging status quo to improve patient management in improving user experience. Each time we develop a product, our passion for innovation, high technology and quality is serving our vision.
PASSION FOR INNOVATION
Over the last 40 years, our development has been driven by innovation, in particular through
Research and Development of unique and revolutionary solution.
The filing of dozens of patents for brands and mechanisms;
A brand new 2800 m2 manufacturing plant located with a 400 m2 clean room class 10, 000 (ISO 7).
PASSION FOR HIGH TECHNOLOGY
The implantation of Sophysa production site in Besançon, capital of watchmaking and high precision is no coincid ! Sophysa has indeed always been driven by the ambition to develop its technological know-how, in areas as diverse as: precision thermoplastic injection molding silicone, treatment thermochemical carbon, encapsulation, the assembly of micro-sensors in silicon, miniaturization, ...
PASSION FOR QUALITY
It first relied on the involvement and the unique skills of our collaborators, whose proven expertise allows them to make and finalize some precision parts manually, according to technics and methods developed and cultivated internally. This is the «made in France» quality that we proudly exports worldwide.

Sophysa is a French industry pioneer specialized in CSF Management, and Neuro Monitoring for Neuro ICU.
Over the last 40 years, in accordance to our commitment, we never stopped challenging status quo to improve patient management in improving user experience. Each time we develop a product, our passion for innovation, high technology and quality is serving our vision.
PASSION FOR INNOVATION
Over the last 40 years, our development has been driven by innovation, in particular through
Research and Development of unique and revolutionary solution.
The filing of dozens of patents for brands and mechanisms;
A brand new 2800 m2 manufacturing plant located with a 400 m2 clean room class 10, 000 (ISO 7).
PASSION FOR HIGH TECHNOLOGY
The implantation of Sophysa production site in Besançon, capital of watchmaking and high precision is no coincid ! Sophysa has indeed always been driven by the ambition to develop its technological know-how, in areas as diverse as: precision thermoplastic injection molding silicone, treatment thermochemical carbon, encapsulation, the assembly of micro-sensors in silicon, miniaturization, ...
PASSION FOR QUALITY
It first relied on the involvement and the unique skills of our collaborators, whose proven expertise allows them to make and finalize some precision parts manually, according to technics and methods developed and cultivated internally. This is the «made in France» quality that we proudly exports worldwide.

CO-1010 Symmetrical 2 Way Tuohy Needle Connectors are used to connect different pieces of catheters, in a shunt.

CO-2010 Asymmetrical 2 Way Tuohy Needle Connectors are used to connect different pieces of catheters, in a shunt.

CO-3010 Tuohy 3 Way Needle Y Connectors are used to connect different pieces of catheters, in a shunt.

CO-LPS50 Luer-Lock 2 Way Tuohy Needle Connectors are used to connect different pieces of catheters, in a shunt.

CR1 Right Angle 2 Way Tuohy Needle Connectors are used to connect different pieces of catheters, in a shunt.

CS1 Symmetrical 2 Way Tuohy Needle Connectors are used to connect different pieces of catheters, in a shunt.

CS2 Asymmetrical 2 Way Tuohy Needle Connectors are used to connect different pieces of catheters, in a shunt.
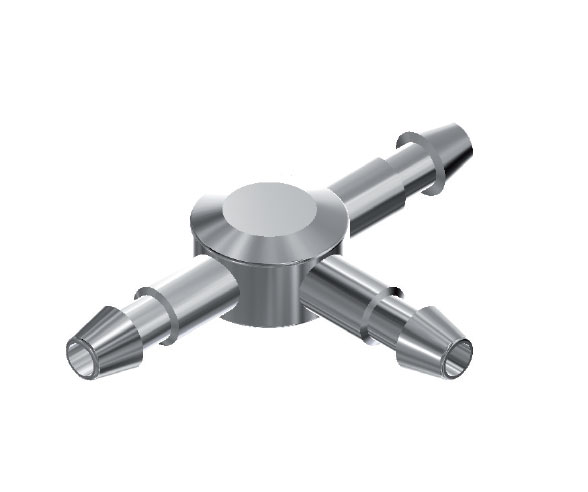
CT1 Tuohy Needle 3 Way T Connectors are used to connect different pieces of catheters, in a shunt.

CO-3010 Tuohy 3 Way Needle Y Connectors are used to connect different pieces of catheters, in a shunt.
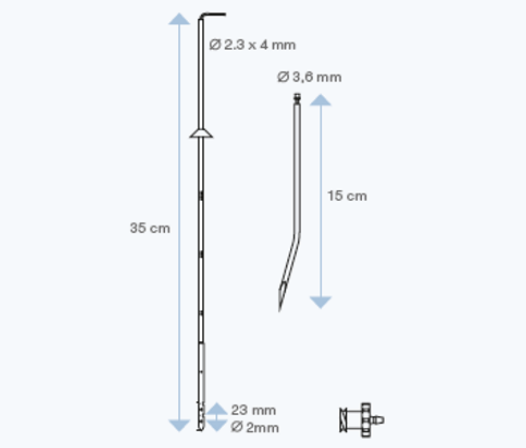
Sophysa offers two ventricular catheters: standard size or large lumen catheter.
Sophysa has developed specific ventricular and lumbar catheters, in radiopaque silicone elastomer, for use with the Sophysa external CSF Drainage Systems.
These catheters allow shunting of CSF from the ventricular cavities or lumbar subarachnoid spaces.
Sophysa offers two ventricular catheters: standard size or large lumen catheter.
Sophysa has developed specific ventricular and lumbar catheters, in radiopaque silicone elastomer, for use with the Sophysa external CSF Drainage Systems.
These catheters allow shunting of CSF from the ventricular cavities or lumbar subarachnoid spaces.
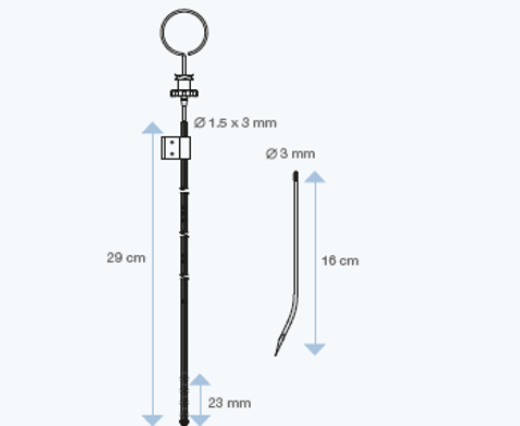
Sophysa offers two Lumbar catheters: standard size or large lumen catheter.
Sophysa has developed specific ventricular and lumbar catheters, in radiopaque silicone elastomer, for use with the Sophysa external CSF Drainage Systems.
These catheters allow shunting of CSF from the ventricular cavities or lumbar subarachnoid spaces.

Sophysa offers two Lumbar catheters: standard size or large lumen catheter.
Sophysa has developed specific ventricular and lumbar catheters, in radiopaque silicone elastomer, for use with the Sophysa external CSF Drainage Systems.
These catheters allow shunting of CSF from the ventricular cavities or lumbar subarachnoid spaces.
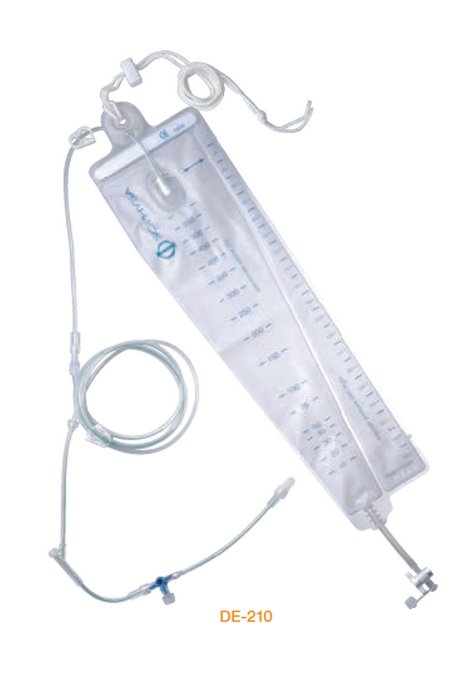
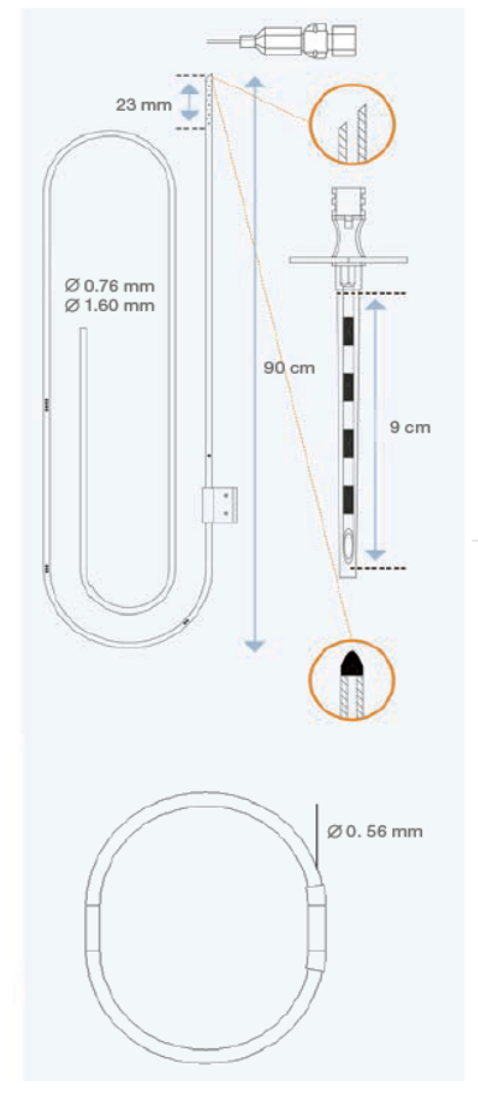
DE-210 External CSF Drainage System. A completely hermetic device, with integral drip chamber, without vent, for decreased contamination risks.
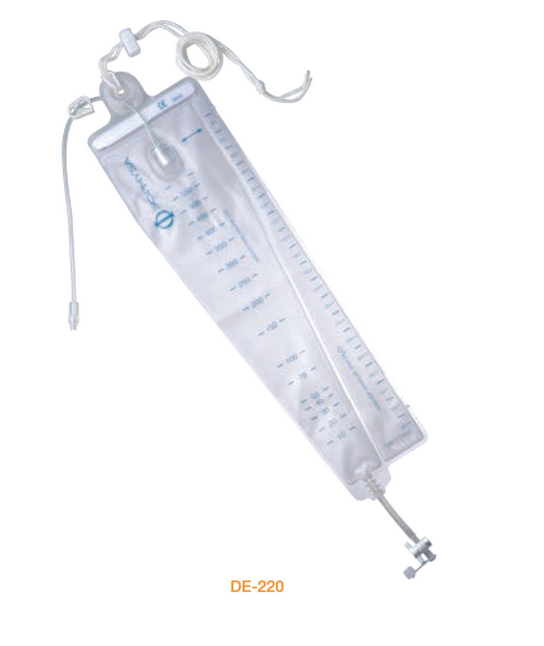
DE-220 External CSF Drainage System. A completely hermetic device, with integral drip chamber, without vent, for decreased contamination risks.
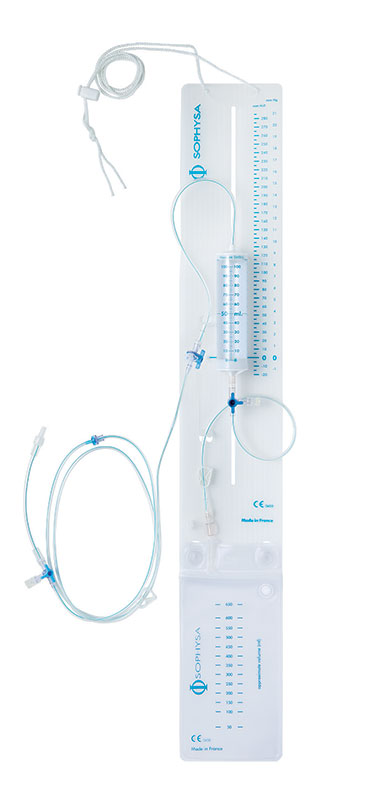
DE-410 External CSF Drainage and Monitoring System with a graduated drip chamber that allows CSF flow rate calculation. This system also permits samples to be taken, medication to be injected and ICP to be monitored.

DE-420 External CSF Drainage and Monitoring System with a graduated drip chamber that allows CSF flow rate calculation. This system also permits samples to be taken, medication to be injected and ICP to be monitored.