
Founded in 1976, SOPHYSA was the pioneer in the development of adjustable neurosurgical valves to treat hydrocephalus. Introduced in 1984, the Sophy® valve was the first adjustable valve in the World allowing non-invasive adjustment of operating pressures, to match an individual patient's changing clinical needs.
Acquired in 1989 by the Japanese tkb Group, SOPHYSA expanded quickly in France, and abroad, gaining global recognition in the field of Neurosurgery.
In 2004, SOPHYSA launched the Polaris® valve, the first adjustable valve with a lock, specifically designed to resist unintentional changes in operating pressure – a milestone in adjustable valve safety. Polaris® offers the patient an unequalled security against the clinical risks associated with those dysadjustments.
In 2005, SOPHYSA introduced Pressio®,a user-friendly Intra-Cranial Pressure monitoring system for neurosurgery and neurotrauma, bringing the continuous intracranial pressure monitoring to a new level of performance and ease of use.
As a neurosurgery specialist, SOPHYSA manufactures and markets a wide range of neurosurgical implants for the treatment of hydrocephalus, including various types of valves, catheters, reservoirs and accessories, a range of ICP sensors and external drainage systems, as well as subcutaneous spinal access ports.
SOPHYSA markets its products worldwide through an international network of exclusive distributors. In France and Benelux, SOPHYSA products are marketed by direct sales representatives.
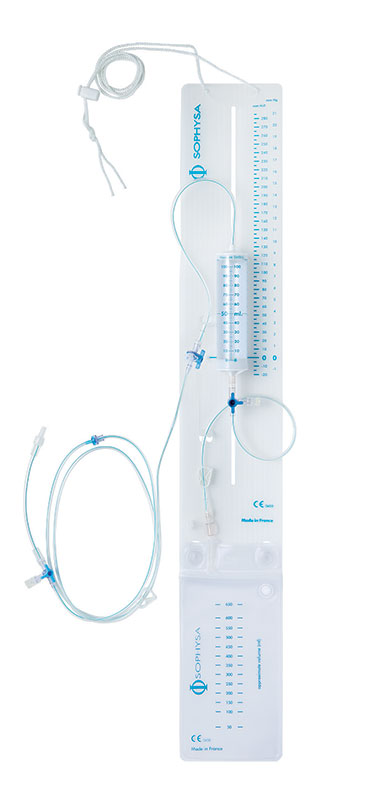
DE-420 External CSF Drainage and Monitoring System with a graduated drip chamber that allows CSF flow rate calculation. This system also permits samples to be taken, medication to be injected and ICP to be monitored.
USER EXPERIENCE & USABILITY
Whatever the medical device is, the final aim of the development process is to provide user a product with an acceptable risk/benefit ratio. On one hand we seek to lower the medical device related risk. On the other hand, we try to reach the highest levels of efficacy.
A common and important point of both elements of this ratio that drives the development of medical devices is the usability.
First, regarding the risk, a recent FDA recall study shown that 44% of medical device recalls are due to design problems, and user errors are often linked to the poor user experience.
On the other side, treating a patient with a medical device frequently requires a more complex sequence of actions than the “simple” delivery of a drug. Consequently the efficacy of a medical device is closely linked to the product usability.
Usability and user experience are the key ingredients to get an acceptable risk/benefit ratio.
OUR VISION
At Sophysa, we believe that usability and user experience of medical devices should never be a brake to patient management. That is why in every development we do, we keep challenging the status quo to provide you a unique user experience enabling you to be totally dedicated to your patient.
Over the last 40 years, in accordance with our vision, we put all our effort in developing ground-breaking solutions improving user experience for a better patient management in the fields of CSF Management and Neuro ICU.
Here are some concrete and well-known example of this dedication to usability :
The Sophy® valvewhich was the first adjustable valve in the World allowing non-invasive adjustment of pressure settings, to match an individual patient's changing clinical needs.
The Polaris® valve, the first adjustable valve with a magnetic lock, specifically designed to resist unintentional changes in pressure settings – a major milestone in adjustable valve safety. Polaris® offers the patient an unequalled security against the clinical risks associated with those dysadjustments.
The Pressio®2 monitoring system, a user-friendly Intra-Cranial Pressure and Intra Cranial Temperature monitoring system for neurosurgery and neurotrauma, bringing the continuous ICP and ICT monitoring to a new level of performance and ease of use.

USER EXPERIENCE & USABILITY
Whatever the medical device is, the final aim of the development process is to provide user a product with an acceptable risk/benefit ratio. On one hand we seek to lower the medical device related risk. On the other hand, we try to reach the highest levels of efficacy.
A common and important point of both elements of this ratio that drives the development of medical devices is the usability.
First, regarding the risk, a recent FDA recall study shown that 44% of medical device recalls are due to design problems, and user errors are often linked to the poor user experience.
On the other side, treating a patient with a medical device frequently requires a more complex sequence of actions than the “simple” delivery of a drug. Consequently the efficacy of a medical device is closely linked to the product usability.
Usability and user experience are the key ingredients to get an acceptable risk/benefit ratio.
OUR VISION
At Sophysa, we believe that usability and user experience of medical devices should never be a brake to patient management. That is why in every development we do, we keep challenging the status quo to provide you a unique user experience enabling you to be totally dedicated to your patient.
Over the last 40 years, in accordance with our vision, we put all our effort in developing ground-breaking solutions improving user experience for a better patient management in the fields of CSF Management and Neuro ICU.
Here are some concrete and well-known example of this dedication to usability :
The Sophy® valvewhich was the first adjustable valve in the World allowing non-invasive adjustment of pressure settings, to match an individual patient's changing clinical needs.
The Polaris® valve, the first adjustable valve with a magnetic lock, specifically designed to resist unintentional changes in pressure settings – a major milestone in adjustable valve safety. Polaris® offers the patient an unequalled security against the clinical risks associated with those dysadjustments.
The Pressio®2 monitoring system, a user-friendly Intra-Cranial Pressure and Intra Cranial Temperature monitoring system for neurosurgery and neurotrauma, bringing the continuous ICP and ICT monitoring to a new level of performance and ease of use.

NNCS1 Symmetrical 2 Way Tuohy Needle Connectors are used to connect different pieces of catheters, in a shunt.

OS03B Bottom Inlet Standard CSF reservoirs are intended for subcutaneous CSF access. All reservoirs are supplied with a separate ventricular catheter.

OS03SSide Inlet Standard CSF reservoirs are intended for subcutaneous CSF access. All reservoirs are supplied with a separate ventricular catheter.
Side-Inlet CSF Reservoirs are flat-bottom reservoirs to be implanted away from the burr-hole.

The safety of adjustable valves has become a major concern for neurosurgeons because of the growing use of electromagnetic devices in daily life and the development of high power MRI (3 teslas).
Indeed, these devices are liable to modify the selected pressure accidentally, with the risk of disrupting CSF drainage and causing serious complications for the patient.
The Polaris® valve is a major breakthrough for the safety of patients fitted with adjustable valves.
Its exclusive locking mechanism enables it to resist:
Direct knocks to the valve;
Everyday magnetic fields;
Magnetic Resonance Imaging (MRI), up to 3 teslas.
It offers the patient an unequalled security against the clinical risks associated with pressure dysadjustments.
The adjustable Polaris® valve is a major breakthrough for the safety of patients thanks to the patented self-locking system of the rotor.
This magnetic lock has been designed to resist unintentional operating pressure changes due to knocks or exposure to magnetic fields, especially during MRI examinations.
It offers the patient an unequalled security against the clinical risks associated with those dysadjustments.
The Polaris® Magnetic Lock
The Polaris® Magnetic Lock is based on the permanent reciprocal attraction of two mobile micro-magnets of opposite polarity.
This “magnetic lock" holds the rotor in the selected position, thus preventing any accidental change in operating pressure if the valve is exposed
to magnetic fields.
In fact, in the presence of a standard magnetic field (unidirectional) the two micro-magnets are attracted in the same direction.
So only one of the two magnets moves in the direction of the field, while the other remains locked.
Changing the operating pressure of the valve first requires the simultaneous unlocking of the two micro-magnets in the valve by a specific magnetic key. The rotor can then turn freely on its central axis.
Normal position
Security position
Valve subjected to a knock or to a magnetic field
Adjustment position
with specific magnetic key
Direct pressure reading is obtained using the Adjustment Kit Compass: the Compass needle is aligned with the position of the magnetic rotor.
In addition to the standard model (30-200 mmH 2O), Polaris® also offers a unique special pressure range :
one low pressure valve and two high pressure valves. Thus a choice is provided, depending on the experience of the practitioner, to meet very specific clinical needs.

RE- 2010 Bottom Inlet Standard CSF reservoirs are intended for subcutaneous CSF access. All reservoirs are supplied with a separate ventricular catheter.

RE- 2011 Bottom Inlet Standard CSF reservoirs are intended for subcutaneous CSF access. All reservoirs are supplied with a separate ventricular catheter.

RE-1021 Side Inlet Standard CSF reservoirs are intended for subcutaneous CSF access. All reservoirs are supplied with a separate ventricular catheter.
Side-Inlet CSF Reservoirs are flat-bottom reservoirs to be implanted away from the burr-hole.

Re-1030 Burr Hole Type Convertible reservoirs are designed to be connected to a shunt, should this conversion become necessary.
For that purpose, they include a silicone lateral catheter closed at its distal end .This outlet catheter may be cut and connected to a valve.

RE-1031 Flat Bottom Type Convertible reservoirs are designed to be connected to a shunt, should this conversion become necessary.
For that purpose, they include a silicone lateral catheter closed at its distal end .This outlet catheter may be cut and connected to a valve.

Re-1141 Single Dome Shunt Reservoir incorporate an outlet catheter designed to be attached to a CSF shunt system.
The outlet catheter, 20 mm long, 1.1 mm ID, 2.2 mm OD, is made of silicone elastomer with a radiopaque stripe. All reservoirs are supplied with a separate ventricular catheter

RE-1241 Double Dome Shunt Reservoir incorporate an outlet catheter designed to be attached to a CSF shunt system.
The outlet catheter, 20 mm long, 1.1 mm ID, 2.2 mm OD, is made of silicone elastomer with a radiopaque stripe. All reservoirs are supplied with a separate ventricular catheter

RE-2021 Side Inlet Standard CSF reservoirs are intended for subcutaneous CSF access. All reservoirs are supplied with a separate ventricular catheter.
Side-Inlet CSF Reservoirs are flat-bottom reservoirs to be implanted away from the burr-hole.

Re-2030 Burr Hole Type Convertible reservoirs are designed to be connected to a shunt, should this conversion become necessary.
For that purpose, they include a silicone lateral catheter closed at its distal end .This outlet catheter may be cut and connected to a valve.

RE-2031 Flat Bottom Type Convertible reservoirs are designed to be connected to a shunt, should this conversion become necessary.
For that purpose, they include a silicone lateral catheter closed at its distal end .This outlet catheter may be cut and connected to a valve.
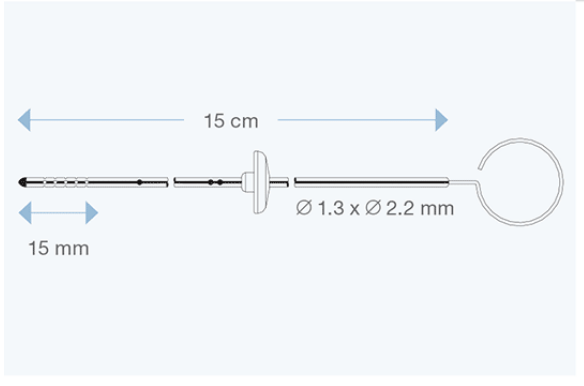
Available in different sizes for both children and adults, they are made of transparent silicone elastomer, with a radiopaque stripe entirely embedded within the silicone wall, and with a tantalum-labelled tip. All models are delivered with right angle adapter and introducing stylet.
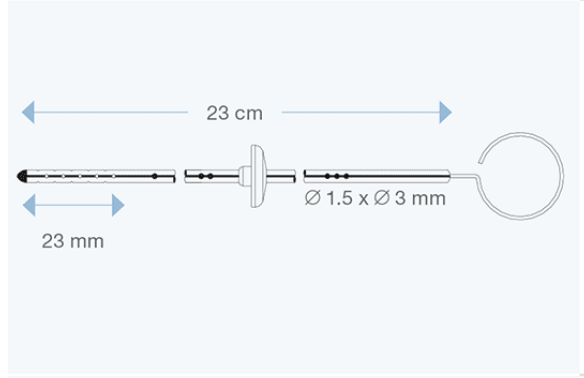
Available in different sizes for both children and adults, they are made of transparent silicone elastomer, with a radiopaque stripe entirely embedded within the silicone wall, and with a tantalum-labelled tip. All models are delivered with right angle adapter and introducing stylet.
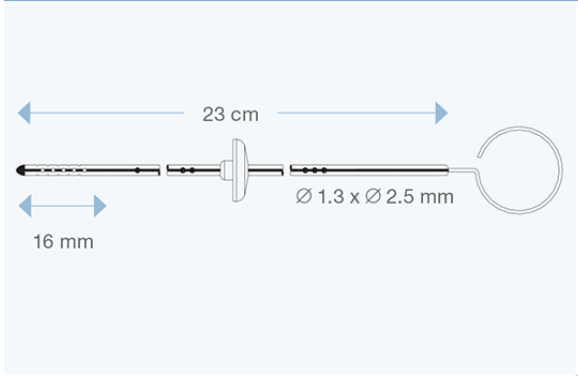
Available in different sizes for both children and adults, they are made of transparent silicone elastomer, with a radiopaque stripe entirely embedded within the silicone wall, and with a tantalum-labelled tip. All models are delivered with right angle adapter and introducing stylet.
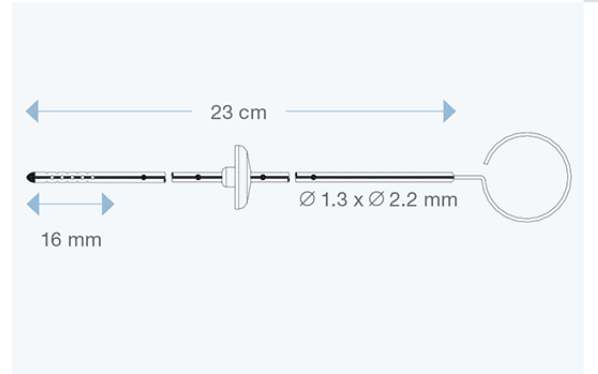
Available in different sizes for both children and adults, they are made of transparent silicone elastomer, with a radiopaque stripe entirely embedded within the silicone wall, and with a tantalum-labelled tip. All models are delivered with right angle adapter and introducing stylet.